Automated, reproducible HLA antibody screening in organ transplants using the MANTIS® Dispenser
Background
The human leukocyte antigen (HLA) system is part of our adaptive immune system and plays an important role in both disease defense and organ transplantation. Donors with matching HLA markers are less likely to reject organs.
Researchers at the Laboratory of Medical Immunology (LMI) at Radboud University Medical Center in the Netherlands screen for HLA-specific antibodies from the sera of potential organ recipients. Detection of antibodies against HLA markers is necessary to avoid hyperacute rejection of the donor organ. Rejection may be caused by blood transfusion, transplantation or pregnancy. The laboratory performs quarterly screenings to detect these harmful antibodies in patient sera.
These antibodies can be detected by cross-matching a patient's serum with a suspension of more than 60 unique lymphocytes from different individuals with different HLA markers. This is also called a complement-dependent cytotoxicity (CDC) test.
In this study, the MANTIS® Liquid Processor from FORMULATRIX® was used to dispense the isolated lymphocytes into the prepared Terasaki plates.
Materials and Methods
First, 1 μl of patient serum was dispensed into a Terasaki plate (Greiner) using an ART Robbins (Sunnyvale, CA) serum dispenser. Donor lymphocytes are isolated from the blood using the density gradient Lymphoprep (Elitech Group) or liquid nitrogen method, then cultured and washed with CFDA C195 (Invitrogen) dye. A lymphocyte suspension of 4 x 106 cells per ml is then prepared.
The MANTIS® Dispenser is used to dispense 1 μL of isolated lymphocytes into the prepared Terasaki plates. Subsequent steps include the addition of suspensions of rabbit complement (Immucore, Norcross, GA) and EDTA (Merck, KGaA, Darmstadt, Germany), hemoglobin and propidium iodide, and incubation.
After the final addition step, microplates were observed under an inverted fluorescent microscope (Leitz Leica Diavert Inverted Microscope withfluorescent light illuminator (480-525nm) Oculair 10x) to determine between patient serum and donor lymphocytes whether there is a positive or negative reaction between patient serum and donor lymphocytes.
The percentage kill (% kill) measurement was determined from the fluorescent staining results (see Figure 1). The percentage kill is the percentage of positive cells (red) to the total number of positive and negative cells (green). The percentage scores are shown in Table 1.
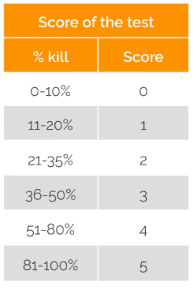
Table 1: Percentage kill score
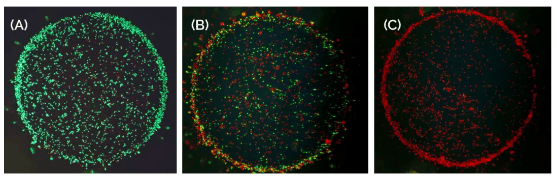
Figure 1: Obviously observable lymphocyte samples (A) Negative reaction, green. (B) Positive reaction, red. (C) Strongly positive reaction, red.
Results and Conclusion
Since the existing equipment is no longer supported, laboratories must find an alternative automated dispenser for adding prepared lymphocytes to Terasaki plates. Manual dispensing introduces a variable amount of variability during the experiment, which is unacceptable for the investigator.
A growing number of laboratories using no longer supported devices for HLA screening will need to replace this outdated technology, so the team at LMI has agreed to share their evaluation for the benefit of the broader academic community involved in this important work.
The MANTIS instrument from FORMULATRIX is considered a superior technique for adding lymphocytes compared to manual pipette spiking and has been successfully used in routine laboratory workflows. It provides results in less time and with higher consistency and accuracy.
Thanks and Acknowledgements
FORMULATRIX® would like to thank Yvonne van Berkel (senior analyst Cellulaire Immunologie en Histocompatibiliteit) and the LMI team for their work in evaluating the HLA workflow of the MANTIS® dispenser and for the data provided in this application note. The results of the data provided in this application note. Other members of the team are Siham Akdimi, Gaby Derksen, Suzanne Hendriks and Jeroen Slager.
Source: @BostonFummerle
Time: 2022.02.21
Related Products
Formulatrix Mantis Microplate Dispenser
Formulatrix USA upgrades micro automated dispensing*
