ECCS B310/B320 Electrochemical reaction visualization confocal system
Japan Lasertec Corporation Battery Electric
With the development of next-generation energy storage devices, lithium-ion batteries are used to power mobile devices and electric vehicles, and demand for them is likely to increase. However, lithium is expensive because it is not an abundant metal. Sodium, on the other hand, is abundant and inexpensive, and interest in sodium ion batteries (SIBs) has been growing. Various materials have been investigated for use as cathodes or anodes in SIBs. Most of these studies have been performed on half-cell systems containing a working electrode (WE), a counter electrode (CE) and, if necessary, a reference electrode (RE). The WE contains either cathode or anode material. Typically, sodium metal sheets are used for the CE in half-cell systems. using sodium as the anode material is conceivable, but this would be difficult in practice due to safety concerns.
There are many reports on electrochemical lithium deposition/dissolution. Lithium metal electrodes have the disadvantage of short circuiting and poor cycling performance due to the formation of lithium "dendrites" during the electrochemical deposition process. Electrochemical lithium deposition in a 0.5 mA/cm2 LiAsF2/ethylene-carbonate-2-methyltetrahydrofuran electrolyte solution, as described in Ref. The lithium grows from the bottom of the lithium electrode and produces kinking. As a result, the shape of the precipitated lithium becomes dendritic. Then, lithium starts to be deposited at the tip of the lithium dendrite and at the kink point. The shape of the deposited lithium is granular. The electrochemical dissolution process of lithium dendrites is as follows. The particulate lithium at the tip and kink points is dissolved. Then, the matrix of the dendrite dissolves and the dendrite becomes "dead lithium". This is one of the reasons for the poor reversibility of lithium metal electrodes. In addition, studies on the shape of electrochemically deposited lithium in various electrolytes have shown that the shape is related to the electrolyte.
As mentioned above, there is an accumulation of reports on electrochemical lithium deposition/dissolution. However, there are few reports on electrochemical sodium deposition/dissolution. It is important to understand the behavior (e.g. shape change, reversibility and coulombic efficiency during cycling) to facilitate the development of SIB. In this study, we focused on metallic sodium as CE and observed the shape change of electrochemically deposited/dissolved sodium in propylene carbonate (PC)-based electrolyte solutions. PC-based solutions are widely used as electrolytes for SIBs, and the use of a single solvent allowed us to elucidate the deposition/dissolution behavior. Sodium and copper electrodes were used as WE and sodium electrode was used as CE as a typical reaction system. For observation, we used an optical microscope as reported in our previous studies on Sn-Co anode materials. This technique allowed us to observe the cross-section of the sodium electrode surface during electrochemical sodium deposition/dissolution and to record videos of the sodium growth for in situ observation. In addition, to investigate the presence of surface thin layers such as solid electrolyte interfaces (SEI) on the deposited sodium, e.g. Li2CO3 and Li2O on the deposited lithium, we examined the surface composition of the deposited sodium using a scanning electron microscope (SEM) equipped with the ionic conductivity of the SEI and other properties important for achieving good recyclability.
In this study, the electrochemical sodium deposition/dissolution behavior in the electrolyte solution based on propylene carbonate was mainly investigated by in situ optical microscopy. First, granular sodium was deposited at the pits of the sodium electrode during the cathodic process. Then, the sodium particles grew linearly from the electrode surface in the form of needles. In the subsequent anodic process, the sodium dissolves near the root of the needles on the sodium electrode and the so-called "dead sodium" is detached from the electrode. The mechanism of electrochemical sodium deposition and dissolution on copper electrodes is similar to that of sodium electrodes.

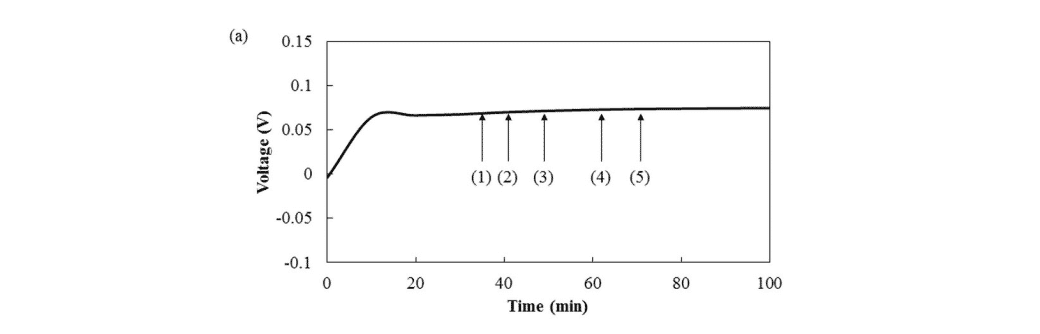
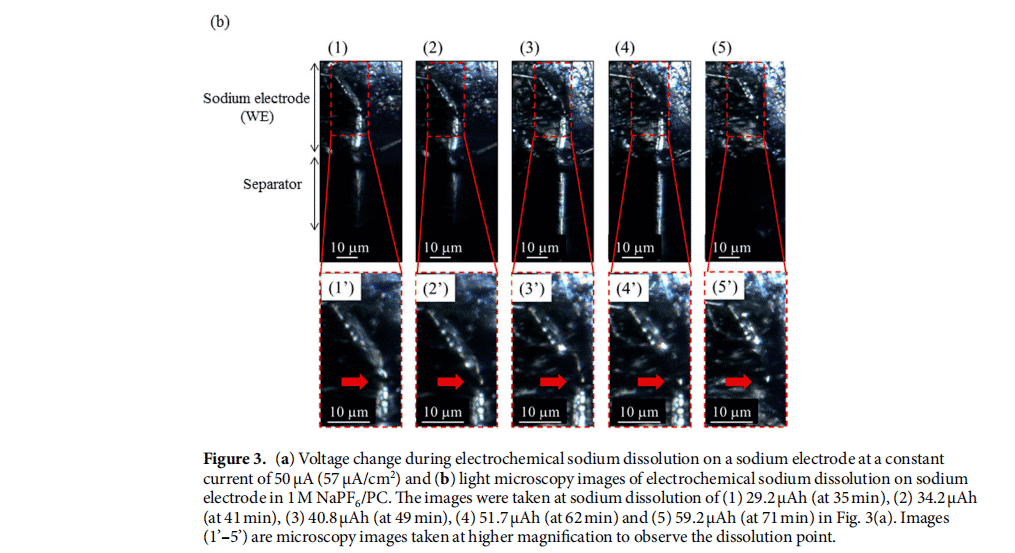

Image 3 shows direct observation of morphological changes in sodium or copper electrodes during electrochemical deposition/dissolution by using an in situ optical microscope (Lasertec Corp., ECCS B310) in the same manner as previously reported. Circular units for microscopic observation were prepared by stacking sodium sheets (0.2-mm thick and 15-mm diameter, Kanto Chemical Corp.) or copper sheets (0.01-mm thick and 15-mm diameter) as WE, electrolyte to prepare solutions (1 M NaPF6/PC, Tomiyama Pure Chemicals Industries Ltd.)-impregnated polypropylene septum (19 mm diameter, Celgard), and sodium flakes as CE. As shown in Supplementary Figure 3(a), the circular cells were cut into semicircles and the exposed cross section of the WE/diaphragm with electrolyte solution/CE of the cell was prepared for observation. The semicircular cell was then placed in a fixture for optical microscopy and the cross-section of the surface was monitored using an optical microscope through a viewing window made of sapphire as shown in Supplementary Figure 3(b). Electrochemical measurements were performed using an automatic constant current discharge-charge system (Hokuto Denko Corp., HJ1001SD8) at a constant current of 50 μA (57 μA/cm2) at room temperature.
Authors: YuhkiYui, Masahiko Hayashi & Jiro Nakamura
Institution: NTT Device Technology Labs., NTT Corporation 3-1, Morinosato Wakamiya, Atsugi-shi, Kanagawa Pref. 243-0198, Japan.; Department of Electronic Chemistry, Interdisciplinary Graduate School of Science and Engineering, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226-0198, Japan. Chemistry, Interdisciplinary Graduate School of Science and Engineering, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226- Nagatsuta, Midori-ku, Yokohama 226-8502, Japan.
Published: received: 24 September 2015; accepted: 12 February 2016; Published: 01 March 2016
Journal: The nature scientificreports
Article source website.In situ Microscopic Observation of Sodium Deposition/Dissolution on Sodium Electrode
Japan Lasertec Corporation Battery Electric